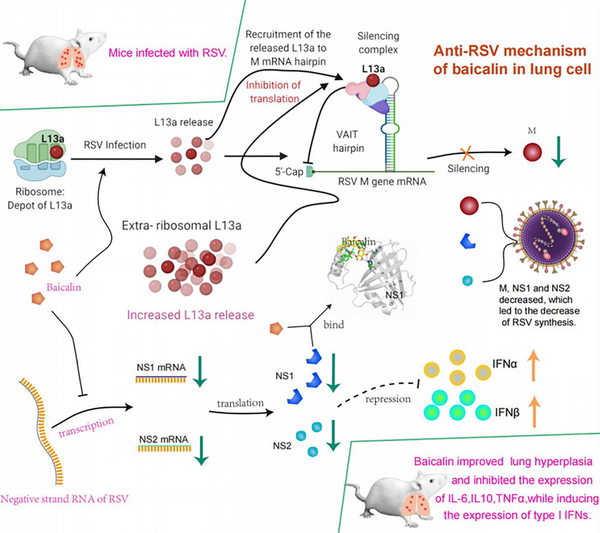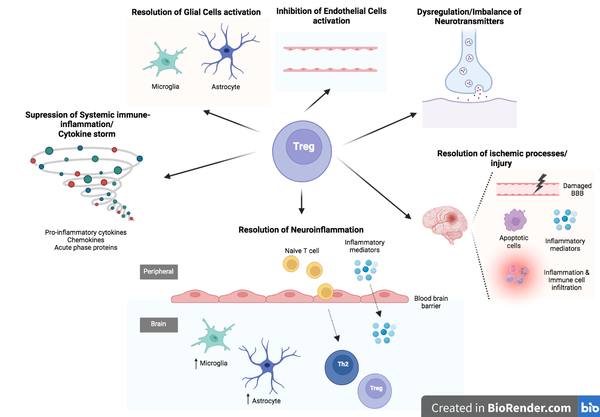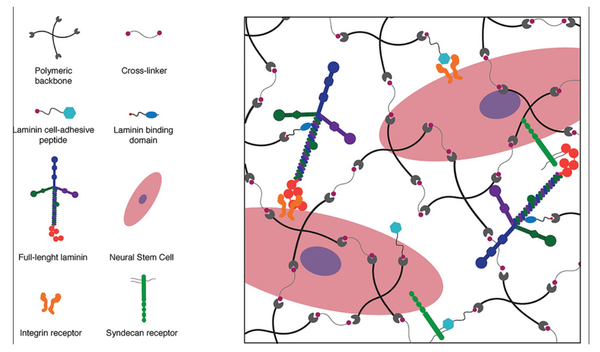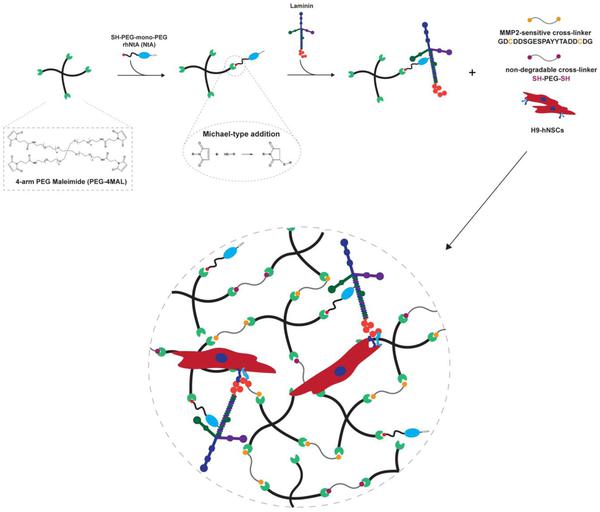


Medical & Science Writer | PhD-trained biomedical scientist
I am a PhD-trained biomedical scientist and medical & scien writer. For the last 10+ years I acquired research skills and writing qualifications that are of great value to create well-tailored and engaging content for the medical and scientific community and the wider public.
I can help you with several projects, including:
- Manuscripts (writing and revision)
- Review articles
- Abstracts
- Grants
- White papers
- Slide decks
- Research protocols
- Literature search and references organisation
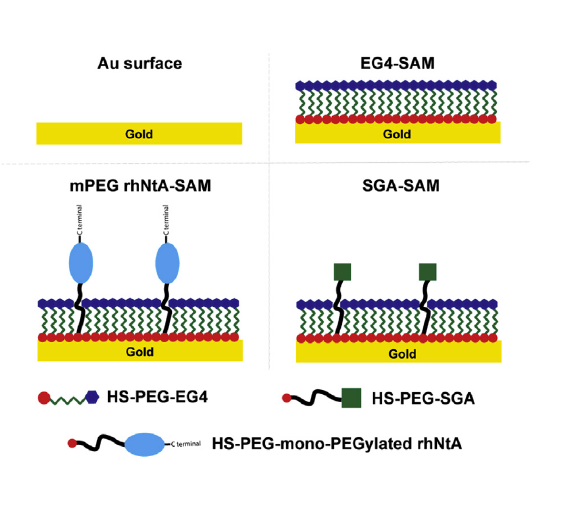
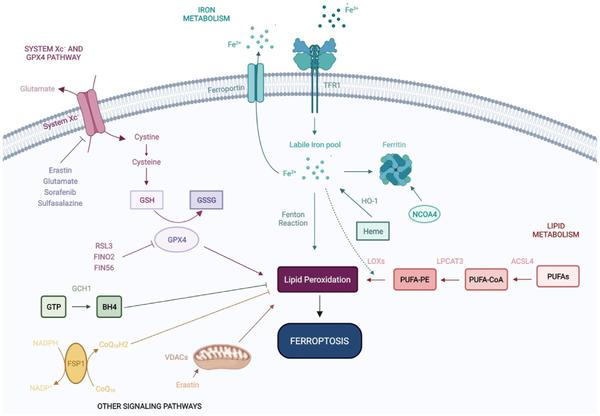
- Scientific Illustration: professional figures and graphical abstracts for publication
- Editing and proofreading medical, academic and scientific documents
My areas of expertise are: Biomedical Engineering, Biochemistry, Cellular & Molecular Biology, and Immunology & Infection
Medical & Science writing support
Authored scientific publications
My inbox is always open, you can contact me with the contact form here